Introduction:
QAC sanitisers are biocidal products which are frequently used for surface hygiene and are prevalent within healthcare. We believe their performance claims risk creating confusion regarding where they should and should not be used. This article takes a closer look at the real science of QAC sanitisers and how we believe this should inform their usage.
What are sanitisers?
Sanitisers are cleaning products with a relatively low level of antimicrobial activity, used for cleaning surfaces and killing certain pathogens. They are often used for institutional surface hygiene across different environments. They take a variety of forms, such as preconcentrate and ready-to-use liquids, dissolvable solids (such as powder sachets) and single-use wipes. The type of biocidal chemical used in most sanitisers for killing certain pathogens is a cationic surfactant, usually a quaternary ammonium surfactant, often referred to as ‘quat’ or ‘QAC’ (quaternary ammonium compound.)
If the marketing is to be believed, QAC sanitisers can effectively clean surfaces and kill pathogens, using persuasive language such as ‘universal action’, potentially leading customers to believe they no longer need to separately clean and disinfect surfaces, which is very tempting. That would be fine if it were true, but we believe this is a misdirection. Given the variety of dangerous pathogens potentially encountered within a healthcare facility, and presence of patients with weakened immune systems, we don’t believe QAC sanitisers should be used in healthcare environments.
Cleaning performance:
Surfactants are primarily responsible for the ability of most cleaning products to remove dirt from surfaces. Because QACs are surfactants, they could potentially achieve cleaning in addition to antimicrobial activity (if enough was used). However, because QACs are more hazardous and expensive than regular cleaning surfactants, the general approach is to use a small amount of a QAC in combination with a larger amount of a secondary surfactant. This helps to minimise the overall risks and costs of a QAC sanitiser. This is feasible, because at low concentrations, although QACs contribute very little to cleaning performance, they can still achieve a usable level of antimicrobial activity (at least to a certain extent).
The QAC therefore only plays the role of the biocide in a QAC sanitiser, and the secondary surfactant plays the role of the cleaner (or that’s at least the intention.) This means a QAC sanitiser requires a second surfactant. Anionic surfactants are usually the most effective surface cleaners, so an obvious choice. However, QACs are incompatible with a wide range of negatively charged chemicals, particularly anionic polymers and surfactants, which when combined, often precipitate out of solution, becoming deactivated and creating residue issues. This means anionic surfactants can’t be combined with QACs in the same product. Instead, nonionic surfactants (which are compatible with QACs, but less effective cleaners) are often used in place of anionic surfactants in QAC sanitisers. This approach inevitably compromises the cleaning performance of QAC sanitisers. This limitation becomes evident when QAC sanitisers are directly compared against far superior, made-for-purpose cleaning products, such as GV Health’s Life Protected Cleaner (LPC), which is a high-level cleaner for removing bioburden from environmental surfaces.
Antimicrobial performance:
QAC Sanitisers are only effective at killing a portion of the dangerous pathogens commonly encountered within a healthcare environment, usually only certain types of bacteria and enveloped viruses1. There are however a wide variety of QAC sanitisers on the market, with varying levels of antimicrobial activity, so we recommend end-users scrutinise their technical documentation to understand their particular capabilities. In order to kill the widest possible spectrum of pathogens responsible for nosocomial infections within the Healthcare environment, a high-level disinfectant is required, such as GV Health’s SoChlor range of Hypochlorous acid releasing NaDCC tablets. If a sanitiser claims it can disinfect, this should set-off immediate alarm bells!
A QAC needs to directly interact with a pathogen’s cell membrane in order to kill it. If a QAC interacts with anything else other than a pathogen, it’s reduced availability limits its antimicrobial performance. QACs have a strong preference to interact with organic dirt, such as humic substances (amongst others)2-3. QACs are therefore reliant on surfaces being clean in order to work effectively. Due to the limited cleaning performance of QAC sanitisers, we are also concerned by the possibility that over time, dirt will gradually accumulate on surfaces, which will increasingly interfere with the QAC, creating a downward spiral of diminishing antimicrobial performance.
Because a QAC’s head group is positively charged, it can strongly interact with any materials carrying a negative charge, including glass, ceramic and cotton4. Cotton is a common component in cleaning cloths. Other materials can develop a negative static (triboelectric) charge, such as untreated wood and various plastics such as PVC and acrylic. All of these materials are commonly encountered within healthcare environments.
In summary, we a concerned that various elements within real-world environments can potentially interfere with QAC sanitisers, namely (i) natural dirt, (ii) applicator cloths and (iii) the surface. Sub-optimal control of pathogens within a healthcare environment means it’s only a matter of time before nosocomial infection rates increase. Exposing pathogens to sub-lethal concentrations of QACs also has the potential to accelerate the rate at which pathogens develop antimicrobial resistance to QACs, of which there is a growing body of evidence5.
Real world relevance of EN certifications for QAC sanitisers:
EN certifications, which are used to substantiate claims of antimicrobial efficacy for sanitisers use artificial dirt to replicate dirty conditions. The artificial dirt is usually Bovine Serum Albumin (BSA), which is a protein. However, BSA only has a mild interfering effect on QACs3,6. QAC based sanitisers are therefore highly unlikely to achieve the antimicrobial activity stated in an EN certification, because real-world dirt, such as humic substances, has a much greater impact on their antimicrobial efficacy than the artificial dirt using in the EN test.
Impact of the COVID-19 pandemic on use of QAC sanitisers:
During the COVID-19 pandemic, QAC sanitisers were proven capable of killing the SARS-CoV-2 virus (from EN 14476 testing), and upon learning this, many suppliers of sanitisers included this information on their product labels. QAC sanitisers therefore experienced a significant increase in usage during the COVID-19 pandemic, further entrenching them within UK Healthcare7.
However, because the SARS-CoV-2 virus is an enveloped virus, it’s relatively easy to kill with a wide array of different biocides. Although claims of effectiveness against the SARS-CoV-2 virus provided reassurance during the COVID-19 pandemic, this shouldn’t be misperceived as ability by extension against other pathogens. Such levels of assurance can only be provided by high-level disinfectants.
Are QAC sanitisers a true 2-in-1 solution for environmental Hygiene?
In some cases, suppliers of QAC sanitisers recommend the product is used twice, with the first stage to remove bulk soiling and second stage to kill certain pathogens. Given the performance limitations of QAC sanitisers, this approach makes sense, but if a two-stage process is required, you may as well use a dedicated cleaner in the first stage and high-level disinfectant in the second stage, which will deliver far superior results on both counts.
Safety concerns:
Some of the most common QACs used in sanitisers carry a number of hazards, some of which are often not seen with standard cleaning surfactants8-13, particularly:
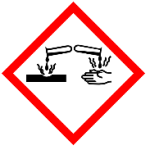
H314 – Causes severe skin burns and eye damage – A category 1 hazard with the following GHS hazard pictograms:

H400 – Very toxic to aquatic life – A category 1 hazard with the following GHS hazard pictogram:
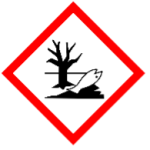
H410 – Very toxic to aquatic life with long lasting effects – A category 2 hazard with the following GHS hazard pictogram:
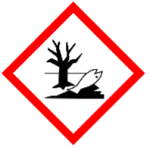
Most QACs need to be used at a concentration of at least 0.1% in a sanitiser to achieve necessary antimicrobial action. Because H400 and H410 hazards are triggered at 0.1% in a formulation (and sometime even lower depending on other factors), this means many sanitisers will incur these hazards, increasing their environmental risk in comparison to standalone cleaning products. The risks associated with handling liquid pre-concentrate QAC sanitisers are further heightened, due to the substantially higher QAC concentration , increasingly the likelihood of triggering additional hazards.
Education on terminology:
The hygiene industry needs to clearly define performance-linked terms such as clean, sanitise and disinfect. Otherwise, end users are at risk of using products interchangeably. Two scenarios of concern come to mind:
Scenario 1 – A QAC sanitiser is used for cleaning:
This approach achieves less effective cleaning than a standalone cleaning product, potentially making cleaning processes take longer, require a greater quantity of product and require higher cleaning frequency. Chemical cleaning occurs immediately during the physical process of wiping or scrubbing with the cleaner. In contrast, antimicrobial action requires contact time, often 5 minutes. Cleaning is therefore a faster process than sanitisation, so using a QAC sanitiser for cleaning unnecessarily prolongs the process, unless the required contact time is not observed, in which case the QAC sanitiser only cleans, therefore achieving the same level of antimicrobial action as a standalone cleaning product whilst delivering inferior cleaning.
The use of QAC sanitisers for cleaning also results in unnecessary exposure to chemicals, which are more hazardous to health and the environment, which is easily avoided by using the correct cleaning product. In summary, the use of QAC sanitisers for cleaning can potentially increases risk, cost and waste compared to standalone cleaner. In this scenario, a dedicated high-level cleaner, such as LPC should be used in place of a QAC sanitiser.
Scenario 2 – A QAC sanitiser is used for disinfection:
This approach won’t work due to the limited antimicrobial activity of QAC sanitisers. The antimicrobial performance of a QAC sanitiser is also susceptible to inference in real-world environments, further undermining it’s capabilities. Because the human eye cannot visually determine whether all dangerous pathogens have been killed, the sub-optimal results achieved by a QAC sanitisers may only become apparent when an outbreak occurs, which is already too late, given the risk this presents to the lives of patients and staff. In this scenario, a dedicated high-level disinfectant, such as SoChlor should be used in place of a sanitiser.
Convenience = more use = safer environments?
Even though QAC sanitisers are less effective than either standalone cleaners or disinfectants, because they seem more convenient, we appreciate staff are more likely to use them, which some may say would lead to improved environmental cleanliness. However, we believe that improving usage frequency should be achieved from the top-down, through better education and training, and ringfencing necessary time for effective cleaning and disinfection rather than using seemingly more convenient, but less effective products.
Conclusions:
In an effort to combine both cleaning and antimicrobial action, QAC sanitisers become a ‘jack of all trades but master of none’ compromising their ability to remove dirt and kill pathogens whilst making them highly susceptible to deactivation in real-world environments, which is not indicated by their EN certifications. We appreciate that the concept of a QAC sanitiser is enticing, because it consolidates two processes into one, which seems more convenient at face value. Although, this is questionable when some QAC sanitisers need to be used twice under certain conditions.
However, in our opinion, when you zoom out and look at the system, QAC sanitisers are at risk of increased risk, cost and waste compared to standalone cleaning and disinfection. Considering the potential for compromised biosecurity due to residual pathogens, and greater hazards during use compared to standalone cleaners, we must ask whether the perceived benefits of QAC sanitisers are justified.
We don’t believe the upfront benefits outweigh the resultant issues created by using QAC sanitisers in healthcare. Some may ask whether they can afford to change from a ‘2-in-1’ QAC sanitiser to a 2-stage process for cleaning and disinfection. However, with surging infection rates from MRSA and C. Difficile in the UK, we suggest the more relevant question to ask is whether they can afford not to14.
| REFERENCES | |
| 1 – | A. Artasensi , S. Mazzotta, L. Fumagalli, Antibiotics 2021, 10, 613. |
| 2 – | S.Fathy, A A El Aal; B. Hunsinger, R. Böhm, Vet. Med.J., Giza, 2008, 56, 115-133 |
| 3 – | P A. Araújo, M. Lemos, F. Mergulhão, L. Melo, M. Simões, Int. J. of Food Sci., 2013, 1-9 |
| 4 – | M. Mali, K. S. Salem, R. Sarder, S. Agate, K. Mathur, L. Pal, Sustainability 2024, 16, 1586. |
| 5 – | P. Pereira, P. Antunes, L. Peixe, A. R. Freitas, C. Novais., Front. Microbiol., 2024, 1-20 |
| 6 – | E. Bessems, Int. Biodeter. & Biodeg., 1998, 41, 177-183 |
| 7 – | P. I. Hora, S. G. Pati, P. J. McNamara, W. A. Arnold, Environ. Sci. Technol. Lett. 2020, 7, 622−631 |
| 8 – | W. A. Arnold, A. Blum, J. Branyan, T. A. Bruton, C. C. Carignan, G. Cortopassi, S. Datta, J. DeWitt, A.-C. Doherty, R. U. Halden, H. Harari, E. M. Hartmann, T. C. Hrubec, S. Iyer, C. F. Kwiatkowski, J. LaPier, D. Li, L. Li, J. G. Muñiz Ortiz, A. Salamova, T. Schettler, R. P. Seguin, A. Soehl, R. Sutton, L. Xu, G. Zheng, Environ. Sci. Technol. 2023, 57, 7645−7665 |
| 9 – | https://chem.echa.europa.eu/100.132.452/overview |
| 10 – | https://chem.echa.europa.eu/100.058.301/overview |
| 11 – | https://chem.echa.europa.eu/100.063.544/overview |
| 12 – | https://chem.echa.europa.eu/100.063.913/overview |
| 13 – | https://chem.echa.europa.eu/100.226.389/overview |
| 14 – | https://www.gov.uk/government/statistics/mrsa-mssa-gram-negative-bacteraemia-and-cdi-quarterly-report/quarterly-epidemiological-commentary-mandatory-gram-negative-bacteraemia-mrsa-mssa-and-c-difficile-infections-data-up-to-april-to-june-2024 |