Introduction:
The UK Healthcare industry is seeing a growing variety of reactive disinfectants used for environmental hygiene, such as peracetic acid and electrolysed hypochlorous acid. Given the science and regulations behind these products, it can be challenging to decide which products to use. Decisions are in-part informed by a product’s claims of antimicrobial performance (bactericidal, virucidal etc.) which according to the biocidal product regulations (BPR), must be substantiated with evidence in the form of EN certifications.
The environments in which reactive disinfectants are used can influence their antimicrobial performance. Dirt and biofilm in particular can significantly diminish the performance of reactive disinfectants, compromising biosecurity. The magnitude of this impact can be staggering, which is discussed in more detail later in this article. However, this consideration is often missing from the conversation, and with the growing range of products on offer, there’s a growing need to discuss it…
Reactive biocides:
The majority of disinfectant products currently available on the market use biocidal chemicals, which are reactive. The direct chemical reaction of the biocide with the pathogen is responsible for killing it. More reactive chemicals are generally more effective disinfectants in principle. The most effective biocidal chemicals used for high-level disinfection are usually oxidising agents (hypochlorite, hypochlorous acid, chlorine dioxide, peracetic acid, hydrogen peroxide etc.). There are however four potential risks commonly associated with their use:
- Reduced stability
- Increased risk to health
- Increase risk of damage to the substrate
- Interference by dirt
All four points warrant discussion, but this article will focus on interference by dirt, due to its impact on antimicrobial performance.
Interference by dirt:
A drawback of reactive biocides is their preference to react indiscriminately with many different materials. Oxidising agents are a good example of this, due to their ability to react with various types of organic and inorganic matter. Organic matter containing nitrogen readily reacts with oxidising agents. The reaction of an oxidising agent is akin to firing a bullet, because the oxidising agent breaks down during the reaction d. i.e. once you’ve used it, it gone.
The dirt which accumulates on environmental surfaces usually contains organic matter, often comprising shed skin cells and hair. These materials contain a significant amount of protein, which is rich in Nitrogen. This means that oxidising agents can react with dirt. If an oxidising agent is consumed by reacting with dirt, it’s not available to kill a pathogen. Because pathogens are microscopic microorganisms which are invisible to the human eye, there is no visible indication to confirm whether an oxidising agent has reacted with the dirt or the pathogen, making customers highly reliant on the product manufacturer’s claims.
Misunderstanding and limitations of EN certifications:
So, we understand that dirt represents a real risk to antimicrobial efficacy of reactive biocides such as oxidising agents. Surely the industry standards recognise this and take this into account during testing? The somewhat unhelpful answer is, sort of.. We have two concerns:
- EN tests can be performed under clean or dirty conditions. But, when a product makes a claim of antimicrobial activity, you often can’t see on the label whether the test was performed under clean or dirty conditions. Consider a scenario where a customer uses a product only tested under clean conditions in a dirty environment – there is no guarantee it will achieve the result it claims.
- The method under which EN certifications test for dirty conditions involves using 0.3% w/v artificial dirt in the form of protein. These conditions are unlikely to replicate the real-world conditions of many heavily soiled environments. This means there is no guarantee that customers will replicate the same results generated in an EN test.
Taking both considerations into account, there is a risk that certain disinfectants may not be suited for certain environment, even though the EN certifications may suggest otherwise.
Biofilm:
In recent years, concerns regarding the contribution of biofilms towards nosocomial infections in healthcare facilities has grown, due to the pathogens they contain, which are capable of being released into the wider environment, for example via splash back from sinks. The ability of biofilm to grow and spread unhindered throughout the drainage and pipe systems of facilities means it can potentially access every part of a facility, further heightening risk1-3. Biofilm represents a particular challenge for reactive biocides.
Biofilms contains a complex mixture of microorganisms and polymers. A portion of these polymers are proteins, which can react with oxidising agents. In essence, biofilm acts as a protective shield against oxidising agents for the pathogens within.
The problem:
Dirt and biofilm can prevent reactive biocides from killing pathogens. Although reactive disinfectants (such as hypochlorous acid and peracetic acid) are highly effective biocides, their performance is substantially reduced in dirty environments.
The challenge:
We need to harness the effectiveness of oxidising agents as effective biocides whilst simultaneously limiting their ability to react with interfering substances such as dirt and biofilm.
Solutions – Cleaning:
The success of a disinfectant used within a healthcare facility is measured by patient outcomes, which is achieved through systems. We must consider all parts of the system to maximise effectiveness. With regards to dirt, the first and simplest solution is to remove it prior to disinfection. For example, GV Heath’s Life Protected Cleaner (LPC) is an ideal pre-cleaning step to reduce bioburden from environmental surfaces prior to disinfection. But we can’t necessarily always guarantee surfaces are thoroughly cleaned beforehand.
Solutions – In-situ delivery of oxidising agents:
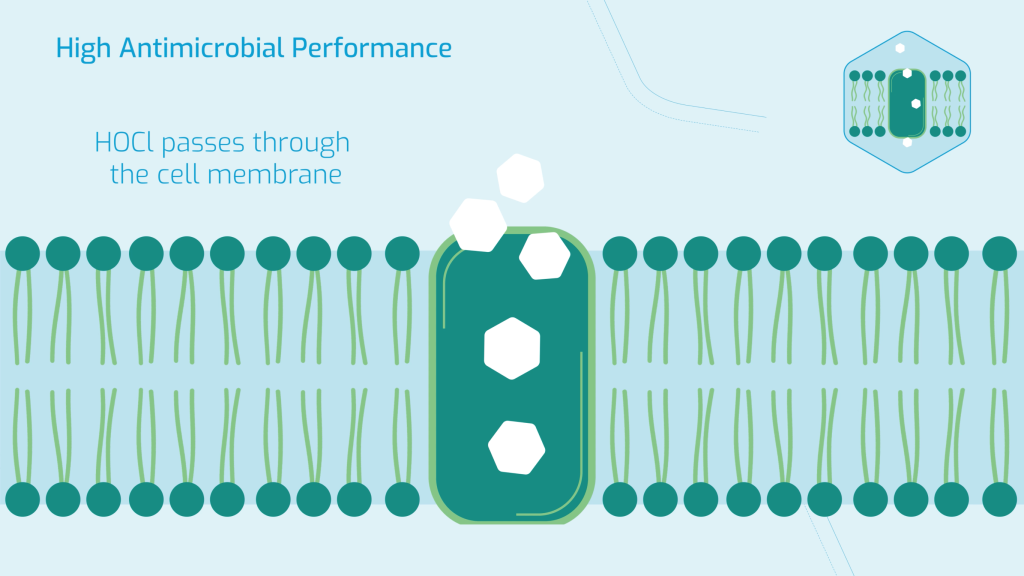
Going back to the analogy of an oxidising agent as a bullet; from a system perspective, we also need to think about how to deliver the bullet to where it’s needed, so we also need to consider the gun. Take Hypochlorous acid for example; it’s an oxidising agent, which is a highly effective biocide, capable of achieving high-level disinfection due to its ability to pass through the cell walls of pathogens and kill them form the inside out. But, as an oxidising agent, it can also react with protein.
We don’t however need to introduce hypochlorous acid directly (from processes such as electrolysis). Instead, we can enclose it in a different chemical called NaDCC (chemical name: Sodium dichloroisocyanurate). If hypochlorous acid is the bullet, NaDCC is the gun. This chemistry forms the basis of all SoChlor products produced by GV Health, which are Hypochlorous acid generating NaDCC tablets. The deceptive simplicity of SoChlor’s tablet format belies the ingenuity of the chemistry within…
NaDCC is not an oxidising agent or biocide. It reacts with water to produce hypochlorous acid in-situ. This reaction is reversible, which is key to its performance. What does this mean? – NaDCC only initially produces a fraction of its total potential hypochlorous acid. Once the hypochlorous acid is consumed by reacting with other materials, it triggers the NaDCC to release more hypochlorous acid.
We theorise that when NaDCC is introduced to a protein rich dirt, although some of the free hypochlorous acid will inevitably react with protein, some of the NaDCC molecules permeate deeper into the dirt before releasing more hypochlorous acid, increasing the probability of hypochlorous acid reacting with the pathogens within the dirt.
This means that NaDCC experiences a smaller reduction in antimicrobial efficacy in the present of protein compared to reactive biocides such as sodium hypochlorite (the active chemical in liquid bleach), which is well documented in peer reviewed academic literature4,5. Said another way, NaDCC is more resistance to interference from dirt compared to other reactive biocides.
For example, Martin et. al. recently published research examining the disinfection of P. aeruginosa biofilm (Infection prevention in practice journal, 2025), with NaDCC and peracetic acid (amongst others). Under these conditions, NaDCC achieved an average log reduction of 8.7, whereas peracetic acid achieved an average log reduction of 3.82. In real terms, this means NaDCC killed 75,000-times more pathogens than peracetic acid under the same test conditions.
With regards to biofilm, we can think of both hypochlorous acid NaDCC as Trogen horses; with NaDCC able to bypass the defences of the biofilm polymers and Hypochlorous acid able to bypass the defences of the pathogens cell walls. These two mechanisms in combination make SoChlor a standout high-level disinfectant technology.
Growing confusion of NaDCC:
We often hear feedback that SoChlor NaDCC tablets and hypochlorite/bleach are the same chemicals, therefore with the same safety, stability, compatibility and performance concerns. Some parties go even further, using the blanket term of ‘chlorine’ to describe any product containing a chlorine based chemical. What potentially follows are scenarios where a party encounters issues with a product such as liquid bleach, views it as ‘chlorine’ and therefore views any other product misidentified as ‘chlorine’ with the same concerns. Given the clear performance benefits of NaDCC over hypochlorite in dirty environments, this is a dangerous misdirection, preventing healthcare institutions from using the most effective tools to safeguard their environments.
To clarify, thousands of different chlorine-based compounds exist which are all chemically and physically distinct from each other. Even when the list of chlorine-based compounds is narrowed down to those capable of direct/indirect oxidation (and therefore capable of biocidal activity), the list is still long (i.e. sodium dichloroisocyanurate, trichloroisocyanuric acid, sodium hypochlorite, calcium hypochlorite, chlorine dioxide, sodium chlorite, sodium chlorate, sodium perchlorate etc.) The obfuscation of the term ‘chlorine’ and the conflation this creates between different chemicals has the potential to steer the healthcare industry away from effective, dependable and proven disinfectants toward less effective alternatives. There are clearly many pitfalls, which can only be avoided with scientifically grounded education, which GV Health is supporting through our growing knowledge centre (https://gvhealth.com/knowledge-centre).
A new term based on known chemistry – molecular dosing:
Within the crucial field of environmental hygiene, ‘dosing’ is a common term, associated with the dilution of liquid chemical preconcentrates to create ready to use cleaning solutions. We can think of NaDCC as the molecular version of an environmental hygiene professional, dosing the active chemical, hypochlorous acid, at the site in which it’s needed. NaDCC achieves molecular dosing of Hypochlorous acid, which elevates it to a substantially higher level of antimicrobial capability than other reactive biocides.
Cleaning and disinfection:
Taking the system one step further, by using GV health’s Life Protected Cleaner to reduce surface bioburden prior to disinfection with GV Heath’s SoChlor range of hypochlorous acid releasing NaDCC tablets, customers have a system on which they can depend to achieve high-level disinfection in any environment.

Growing needs for more testing:
We need to develop new methods to test the efficacy of reactive biocides under conditions which are more representative of real-world healthcare environments. Biofilms represent a particular challenge to replicate in the laboratory. There are currently no tests standards approved in Europe for testing the ability of disinfectants to kill biofilm pathogens. A common approach is to generate biofilm with a CDC biofilm reactor, but this requires the handling of pathogens, limiting its availability to laboratories equipped to deal with pathogens6.
GV Health is currently investigating the creation of synthetic biofilm from chemical raw materials to model the oxidative performance of biocides within biofilm environments as an early-stage validation protocol. Initial results indicate the ability for NaDCC to permeate deeper within biofilms before releasing hypochlorous acid compared to reactive biocides. We intend to showcase this methodology at infection prevention 2025, Brighton and use it to demonstrate the performance benefit of SoChlor over other reactive disinfectants.
References:
| 1 – | C. Hayward, K. E. Ross, M H. Brown, M. A. Nisar, J. Hinds, T. Jamieson, S. C. Leterme, H. Whiley, Sci. of the Tot. Env. 2024, 949, 175194 |
| 2 – | L. O. Parkes, S. S. Hota, Current Infectious Disease Reports, 2018 20:42 |
| 3 – | J. Maillard, I. Centeleghe, Antimicrobial Resistance & Infection Control, 2023 |
| 4 – | S. F. Bloomfield and E. E. Uso, Journal of Hospital Infection, 1985, 6, 20-30 |
| 5 – | D. Coates, Journal of Hospital Infection, 1988, 11, 60-67 |
| 6 – | D. L. Williams, S. R. Smith, B. R. Peterson, G. Allyn, L. Cadenas, R. T. Epperson, R. E. Looper, PLOS ONE, 2019. |